Describe How a Calorimeter Measures Heat
The modern Bomb calorimeter is a development of the original calorimeter of Berthelot. The temperature of the water is measured with the thermometer.
But this time the calorimeter is continually measuring the temperature of the water.

. The modern bomb calori. The calorimeter traps all the heat from a chemical reaction we measure the effect of that heat on the temperature of water in the calorimeter and we can then calculate the heat energy released by the reaction. The modern Bomb calorimeter is a development of the original calorimeter of Berthelot.
View the full answer. When a reaction occurs in an aqueous solution the reaction is siad to occur at constant pressure atmospheric pressure. A thermometer is used to measure the heat change in the amount of water.
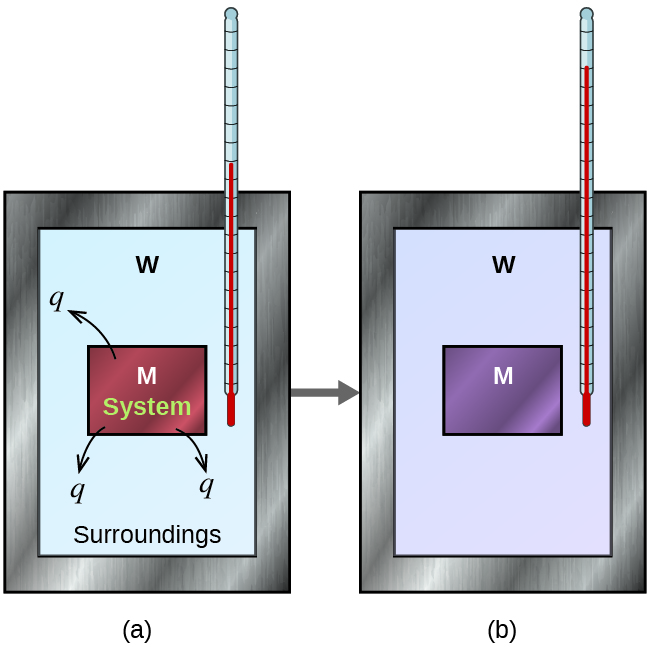
It can be measures by using a calorimeter. The calorimeter is an insulated container in which we place a measured mass of water. Delta T is change in temperature.
Energy can be released in chemical reactions as light sound or electrical energy. A calorimeter is an object used for calorimetry or the process of measuring the heat of chemical reactions or physical changes as well as heat capacityDifferential scanning calorimeters isothermal micro calorimeters titration calorimeters and accelerated rate calorimeters are among the most common types. Calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
It mainly consists of a metallic vessel made of materials which are good conductors of electricity such as copper and aluminium etc. But it is most often released as heat energy. ARC method measures the heat generation rate of battery based on the increase of batterys temperature and the amount of heat released from the.
By measuring the temperature change of the water with a thermometer the amount of heat the. Battery is preferably measured directly using a calorimeter. Describe how a constant volume calorimeter can be used to measure the heat transferred from a burning piece of coal to cool water.
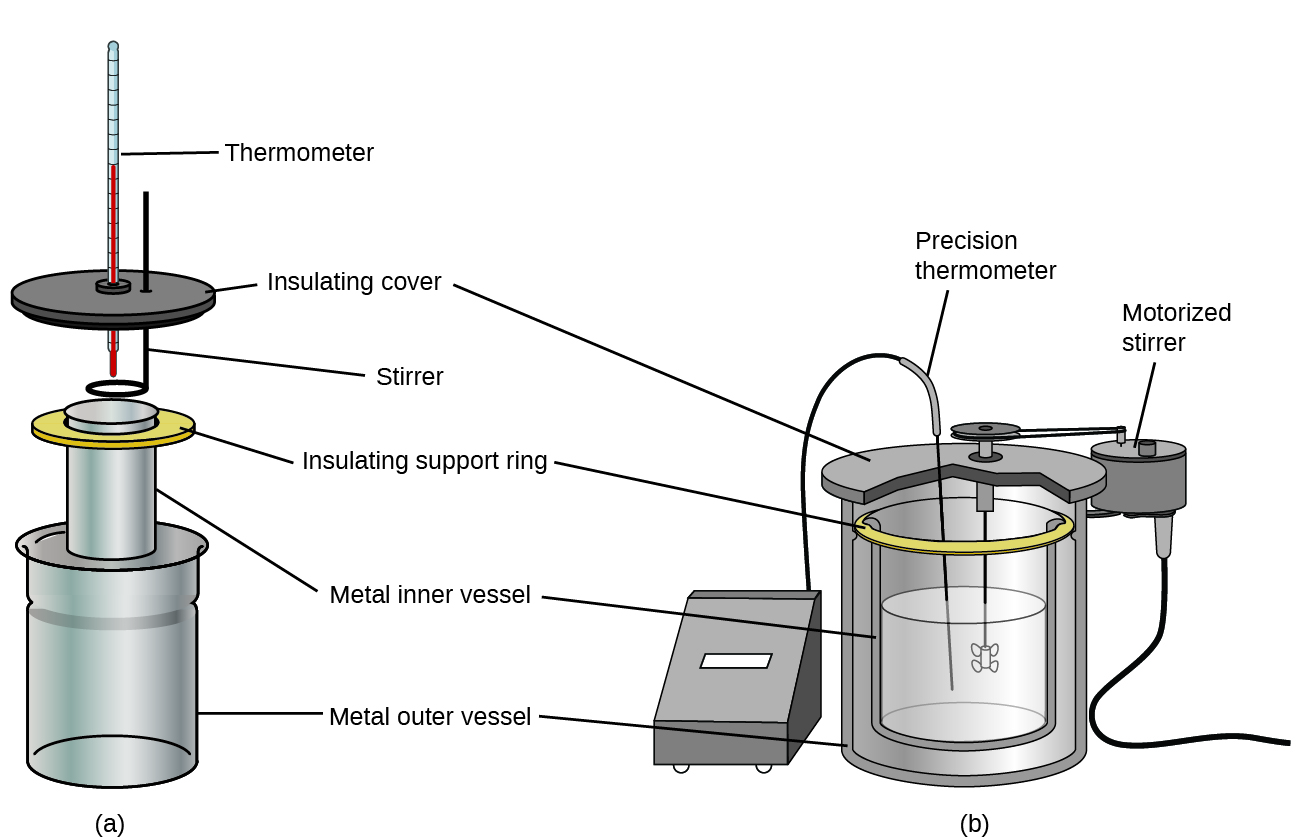
Heat is leaving the water and going into the ice causing it to melt so. Calorimeters include two vessels. There are two known methods.
Ad Over 27000 video lessons and other resources youre guaranteed to find what you need. The water in the calorimeter cup and the object exchange heat until they are at the same temperature. A calorimeter is a device used for heat measurements necessary for calorimetry.
Calorimetry - measuring energy changes from combustion. The specific heat of water is different from the specific heat of ice and so wet ice into a calorimeter experiment can increase the mass of water in the calorimeter and become a. The calorimeter is filled with fluid usually water and insulated by means of a jacket.
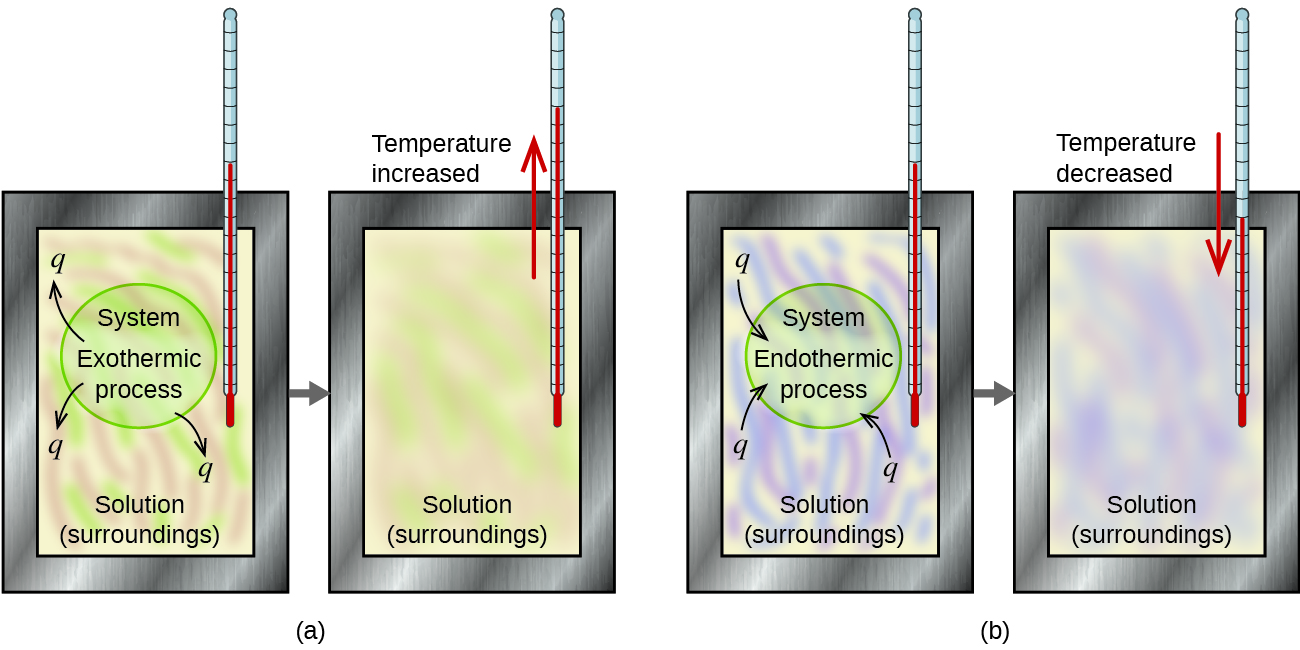
Another object at a different temperature is dropped into the water. The calorimeter is well insulated and a thermometer is built into the calorimeter to precisely measure the temperature of the water inside. For example when an exothermic reaction occurs in solution in a calorimeter the heat produced by the reaction is absorbed by the solution which increases its.
If we were to then put ice into the water it would meltjust like in the coffee example. Simple calorimeters are made with a metal container of water positioned above a combustion chamber. A calorimeter is generally used to measure the amount of heat energy and then uses that to calculate the specific heat of a substance or other heat related information.
Want a similar task completed for you. Reiterate the major experimental data. The specific heat of water is a well-known quantity 418 Jg-o C.
Accelerated rate calorimetry ARC and isothermal heat conduction calorimetry IHC 9. Then the heat liberated or absorbed is the heat of reaction at constant pressure Delta H. A calorimeter measures the change in heat.
State the heat of formation for MgO and describe any errors that may have led to an incorrect value of either the heat of formation Mg2 or MgO or the calorimeter constant. Because the chamber is well insulated the heat produced by the body is absorbed by a known volume of water that circulates through pipes located in the chamber. The calorimeter is filled with water.
C is the specific heat. 19 To obtain these measurements a subject is placed in a sealed chamber with a supply of oxygen. One is known as an outer vessel and the other is known as an inner vessel.
The bomb calorimeter is an instrument used to measure the. The calorimeter used to determine the energy change during a reaction accurately is known as a bomb calorimeter. Previous question Next question.
The calorimeter used to determine the energy change during a reaction accurately is known as a bomb calorimeter. Direct calorimetry measures heat production or heat loss by the body. Let us take the example of enthalpy of neutralisation of an acid by a base.
The completed Excel file. Calorimetry is the science of measuring the heat flow into or out of a system for chemical or physical changes It is a method to measure the heat effect of a processwhich could be physical changes such as melting evaporation dehydration could also be defined as chemical change or it can be a chemical change such as acid-base neutralization dissolving solid-state. A simple calorimeter just consists of a thermometer attached to a.
From the change in temperature the heat of reaction can be calculated. There is also a facility for stirring the contents of the vessel. Once the sample is completely combusted the heat released in the reaction transfers to the water and the calorimeter.
The modern bomb calorimeter is made of corrosion resisting steel in which the combination Bomb Calorimeter. The amount of heat absorbed or released by a substance as its temperature changes is calculated using the equation qcmdeltaT where c q is the heat. The air between the inner vessel and the outer vessel works as a heat insulator so there is no or very.
No comments for "Describe How a Calorimeter Measures Heat"
Post a Comment